Gas Sensors types and mechanism
There are various methods of gas sensors, and it is important to select the most suitable gas sensor according to the gas to be measured and the application. Typical gas sensor methods include a non-dispersive infrared (NDIR) method, a semiconductor method, and an electrochemical method.
This section explains the principle of each gas sensor's detection methods and its advantages and disadvantages.
CO2 Sensors
Types and Characteristics of Gas Sensors
Depending on the detection principle of the gas sensor, the gas to be detected differs. Also the sensors have advantages and disadvantages in terms of performance. The chart below compares the three typical detection methods (the ✓ has advantages and the ✗ has disadvantages).
Table 1. Types and Characteristics of Gas Sensors
| NDIR method (Non-dispersive infrared method) |
Semiconductor method | Electrochemical method | |
|---|---|---|---|
| Principle | Detection method using infrared absorption of gases |
Detection method of absorbing gas onto the metal oxide surface |
Detection method of oxidation-reduction reaction of gas at the sensing electrode |
| Precision | ✓ | × | × |
| Current consumption | ✓ | × | × |
| Life time | ✓ | × | × |
| Response time | ✓ | × | × |
| Size | ✓ | × | × |
| Gas to be measured | Carbon dioxide Flammable gas Refrigerant gas Exhaled alcohol |
Flammable gas Refrigerant gas VOC (Volatile Organic Material) |
Toxic gas |
NDIR method
A NDIR gas sensor uses the property that gas molecules absorb infrared rays at specific wavelengths. CO2, which is a typical gas, is 4.3um, while the refrigerant-gas R32 absorbs infrared radiation in the 3.3um band. This NDIR method consists of a light emitter, an infrared sensor, and an optical cavity. The infrared rays emitted from the infrared light source are absorbed by gas molecules in the optical cavity and reach the infrared sensor after being attenuated. Based on the difference in the amounts of infrared radiations reached, the gas concentration can be calculated (figure 1).
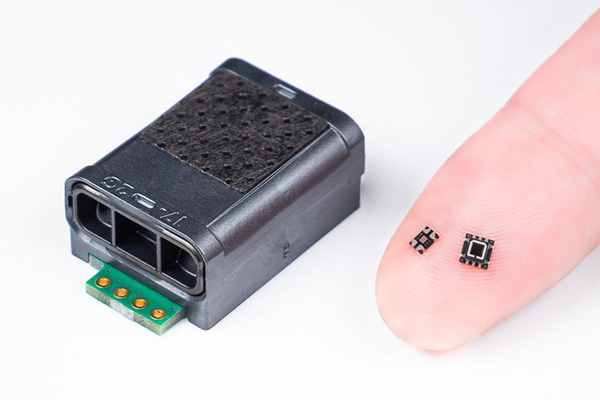
The figure 2 shows the behavior of the infrared sensor with respect to the gas concentration at that time. The higher the gas concentration, the more infrared light is absorbed by the gas molecules, consequently reducing the infrared sensor output. On the other hand, the lower the gas concentration, the higher is this output figure. The gas concentration can be found by calculating this output based on Lambert-Beer's Law.
 Figure 1. Principle of NDIR method
Figure 1. Principle of NDIR method
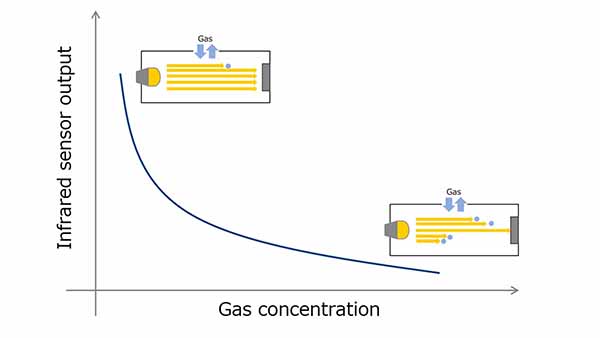 Figure 2. Gas concentration vs. infrared sensor output
Figure 2. Gas concentration vs. infrared sensor output
The NDIR method is superior to other methods in terms of measuring gas concentrations due to its ability to detect inert gases such as CO2. This lack of detection is the weak point of other gas sensing methods. The NDIR method is capable of precision measurements regardless of whether the gas is active or inactive, as long as it has absorbance in the infrared region. On the other hand, hydrogen and oxygen cannot be measured because they have no absorption in the infrared region due to their symmetric molecular structure. In addition, NDIR gas sensors, which are equipped with a light emitter and an infrared sensor, achieve ultra-low power consumption, ultra-fast response, and a high safety level.
More information on the NDIR method is provided here.
Semiconductor method
Oxygen absorbed on a metal oxide that is heated (>300°C) reacts with the gas to be detected, thereby changing the sensor resistance value. The semiconductor gas sensor uses this property.
N-type metal oxides, such as tin oxide and zinc oxide, are used for the metal oxide where gas is absorbed. Since such a metal oxide can be produced by semiconductor process, semiconductor gas sensors can be mass-produced easily and therefore, economically.
At the same time, since a sensor of this method requires absorption of a gas onto a metal oxide surface, this method is unsuitable for detecting chemically stable gas molecules such as CO2 and has a disadvantage such as limitation of the measurable gases.
Electrochemical method
Electrochemical gas sensors use oxidation-reduction reactions to measure gas concentration. The gas molecules to be detected undergo an oxidative reaction at a sensing electrode, generating ions and electrons. Ions are transferred to the counter electrode via an electrolyte and electrons are transferred to a counter electrode via an external circuit, resulting in a reduction.
CO is one of the typical gases used for measurement and CO2 is generated as a result of the reaction of CO with a sensing electrode. The electrochemical sensor uses this chemical reaction. At this time, since the current flowing through the external circuit increases in proportion to the gas concentration, the gas concentration can be calculated by monitoring the current value.
On the one hand, the sensors have the advantages of not being interfered by other gases and being resistant to condensation, but on the other hand, the sensors are said to have a short life span and require regular maintenance.
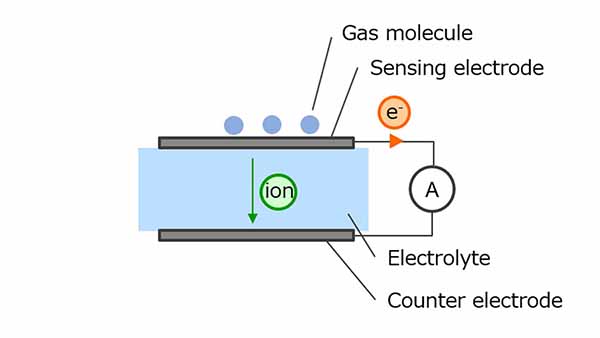 Figure 4. Electrochemical method gas sensor
Figure 4. Electrochemical method gas sensor
CO2 Sensors
Flammable Gas Sensors
About Senseair
Senseair, which became a member of the Asahi Kasei Microdevices (AKM) Group in 2018, is a provider of gas sensors using NDIR: Non-Dispersive InfraRed technology. Our goal is to constantly develop and mass-produce new gas sensor technologies.